The Silver Mirror Test
By Marco Wong
(Published On 19 November 2021)
Chemical experiments do not always end with appealing products but this reaction produces a shiny silver mirror effect to the glass surface! Readers who study organic chemistry might have seen this experiment already, it is the chemical test we use to distinguish between aldehydes and ketone functional groups known as the Tollens’ test, or ‘silver mirror test’, which is developed by Bernhard Tollens.
Tollens’ reagent
The Tollens’ reagent consists of a solution of silver nitrate and ammonia, along with sodium hydroxide to maintain alkaline pH. Under alkaline conditions, the main component of the reagent, diamminesilver(I) coordination complex, is formed. Since coordination complexes are not very stable, it has a short shelf life, therefore it needs to be freshly prepared prior to the experiments.
To prepare the reagent, sodium hydroxide is add to the silver nitrate solution to form silver(I) oxide precipitate:
![]()
Then ammonia is added until all the brown precipitates dissolve:
![]()
The result is a diamminesilver (I) coordination complex stabilized in an alkaline solution.
A Redox reaction
The silver mirror test is a redox reaction, where the [Ag(NH3)2]+(aq) is reduced to Ag(s). The oxidation number of silver is reduced from +1 to 0 in the process. The diammine complex allows the silver ligand to react more readily, therefore the silver nitrate solution cannot produce the same effect. Since aldehyde can be oxidised but ketone cannot, mixing aldehyde with Tollens’ reagent would yield a positive result of a layer of silver metal deposit on the container surface.
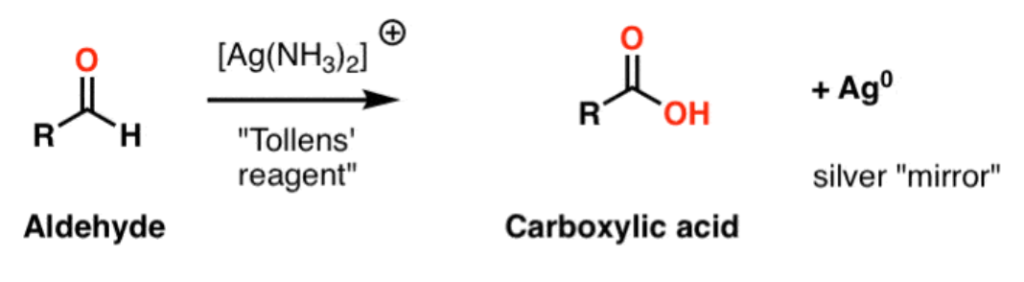
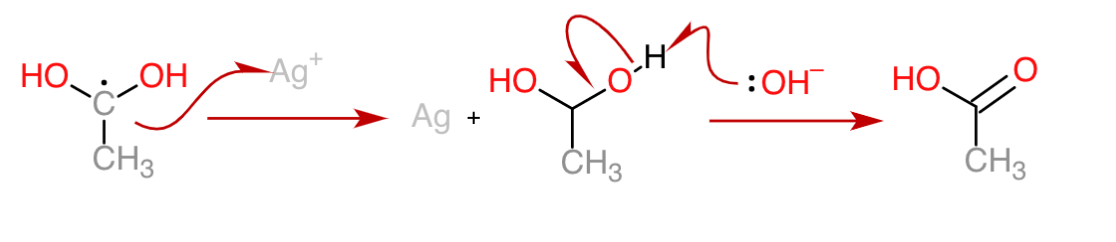
If you are still reading, you are probably interested in the mechanism in organic chemistry, the following are the steps of the reaction of aldehyde in Tollens’ test:
1.The electron is transferred from the carbonyl group to an Ag+ ion, depositing a silver atom and forming a carbocation.
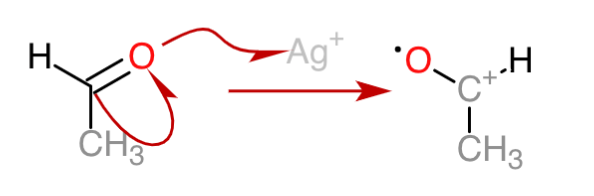
2. The carbocation attracts a hydroxide ion and the hydrogen is shifted, forming a carbon radical.
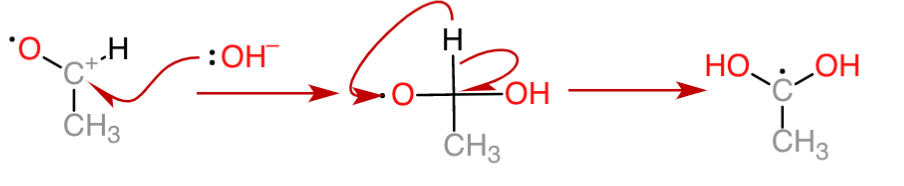
3. The carbon transfers the electron to another silver ion, forming carbocation again. Then a hydroxide ion provides the electron for the formation of a carbonyl bond.
Applications
The reaction is now widely used in laboratories and is taught as a chemical test to distinguish aldehyde and ketone. The effect of leaving a silver mirror surface in the inner surface of the container makes this appealing experiment not limited to the laboratory. In fact, the very same silver plating method was invented in 1835, by a German chemist Justus von Liebig, to produce silver mirrors. Normally, an insulator like glass cannot be electroplated very easily, it requires a stable electric power supply and some special treatments. This silver plating method provided a better solution when electricity wasn’t as readily available.
Although we might not need to make mirrors by the silver mirror reactions anymore. Some glassware workshops still use the reagent to produce beautiful shiny Christmas ornaments! Check out this video to see the creation of these festive silver globes:
Marco’s teaching style is interactive and communication-focused; he focuses on motivating students to be active learners. Sign up a trial lesson with Marco now!
About The Edge
The Edge Learning Center is Hong Kong’s premier Test Preparation, Academic Tutoring, and Admissions Consulting services provider. Founded in 2008, The Edge has helped thousands of students improve their ACT and SAT scores as well as their IB and AP grades. The AC team has just finished off another successful period in which students gained acceptance to schools such as Columbia, Yale, UChicago, and more! Check out our latest Admissions Results!

