Chemistry How To: Electrochemical Cell
By Marco Wong
(Publish On 28 June 2021)
With the exam in May coming up, students should be practicing hard and honing the skills.
In redox reactions, the more advanced questions will be regarding voltaic cells and electrolytic cells. Although the questions vary a lot, the reactions only comprise several things : anode reaction, cathode reaction and electrolyte. The key to mastering electrochemical cells, or redox in general, is to identify them accurately and quickly.
For both voltaic cells and electrolytic cells, reactions only occur at the electrodes, where there is an gain/loss of electrons between the electrolyte and the electrodes. Most importantly, reduction occurs at cathode and oxidation occurs at anode, for both voltaic cells and electrolytic cells.
Mnemonic : “Red cat, an ox”

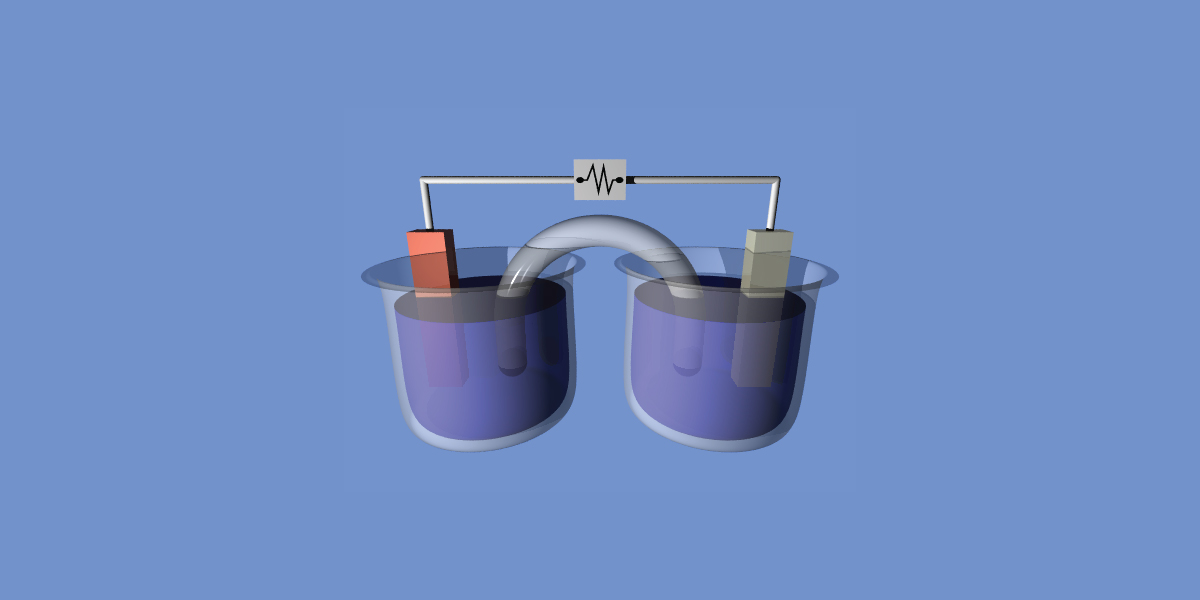
Voltaic Cell
 As the name implies, the electrochemical cells generate voltage. In HL, the voltage is calculated by the difference between the standard cell potential. Most of the voltaic cells seen in IB consist of two half-cells, where a metal electrode is placed in a solution containing ions of the same metal. The half cell of copper represented by:
As the name implies, the electrochemical cells generate voltage. In HL, the voltage is calculated by the difference between the standard cell potential. Most of the voltaic cells seen in IB consist of two half-cells, where a metal electrode is placed in a solution containing ions of the same metal. The half cell of copper represented by:
Cu(s)|Cu2+(aq)
The vertical line represents the phase boundary. To complete a voltaic cell, the two half cells must be connected by a salt bridge of inert ions. The completed voltaic cell can be represented by:
Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
Now let’s look at this example, by convention, the anode is typically written on the left hand side. Let’s ignore the convention and try to determine the anode and cathode first.
By definition, oxidation must occur on the anode. Zn(s) is a stronger reducing agent than Cu(s), therefore the oxidation reaction must be:
Zn(s) → Zn2+(aq) + 2 e–
From there we can also deduce the reduction reaction:
Cu2+(aq) + 2e– → Cu(s)
So in the voltaic cell, Zn is the anode, and Cu is the cathode.
A common question would be suggesting a suitable salt bridge for the cell. Anode attracts anion; cathode attracts cation. The key is to find an anion that does not precipitate in the anode electrolyte and a cation that does not in the cathode electrolyte. In this case, sodium sulphate would be a valid answer.
Observable changes:
- colour changes as the metal ions concentration increases/decreases
- Mass increase at cathode/decrease at the anode
Electrolytic Cell
Contrary to the voltaic cell, the reaction is not spontaneous. Energy, or a voltage, is applied to the cell to make the reactions occur. Electrolysis is a very useful technology in industrial chemistry, example uses include extraction of metal and electroplating.
Since the reactions are driven by only the current supplied, anode and cathode are defined by the energy supply. In SL, simply look at the direction of electron flow, the side connecting to the anode of the battery/DC supply is the cathode of the electrolytic cell. HL students will need to check if the voltage is high enough to overcome the standard cell potential (worry not! This is usually provided by the question).
Molten salt electrolytic cell:
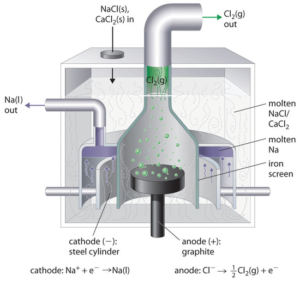
The shapes of the cells might vary depending on the questions, but the reaction would always be the same. Molten salt is highly conductive because of the mobile ions. In most of the electrolysis examples, the reaction is focused in the electrolyte/molten salt and therefore inert electrodes are used. Platinum is an ideal electrode but is also expensive, graphite on the other hand is a good substitute.
In this example, anode is where the oxidation occurs, in the mobile phase, there are only Na+ ion and Cl– ion, thus the oxidation is 2Cl– → 2Cl(g) + 2e–. Similarly, the reduction reaction is Na+ + e– → Na(s).
Electrolysis of aqueous NaCl
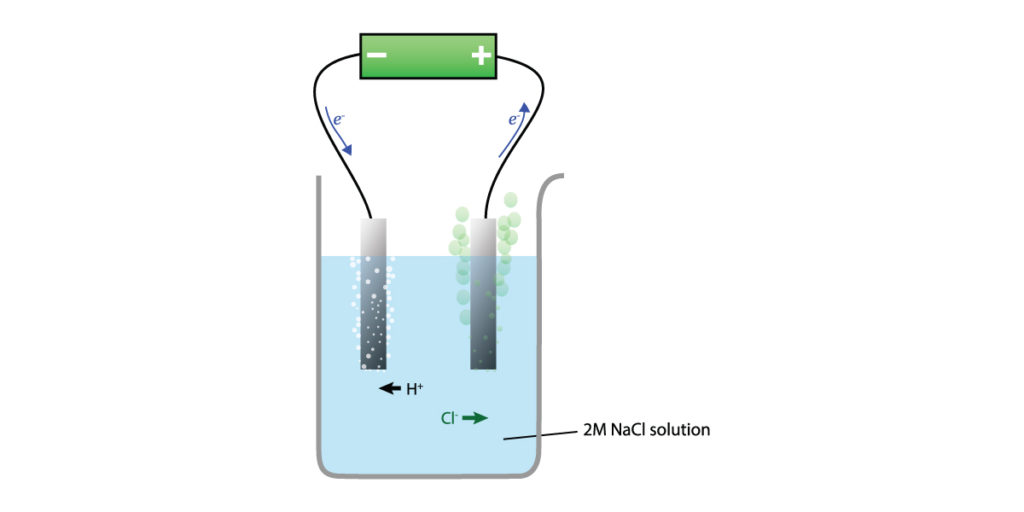
In aqueous solution, H+ and OH ions can also undergo redox reactions, therefore they must be taken into account. H+ is a stronger oxidizing agent than Na+; Cl– is a stronger reducing agent than OH–. Depending on the concentration of NaCl, there are different products of electrolysis.
Brime (concentrated NaCl solution)
The reactions are:
2H+(aq) + 2e– → H2(g)
2Cl–(aq) → Cl2(g) + 2e–
Diluted NaCl solution
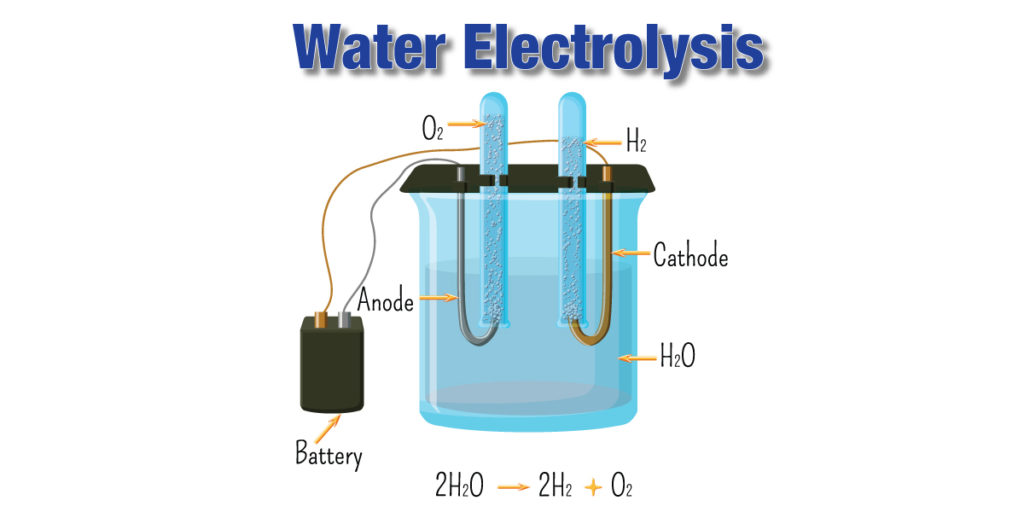 In a very dilute solution, the presence of ions is to increase the conductivity of water. The actual reaction is the electrolysis of water.
In a very dilute solution, the presence of ions is to increase the conductivity of water. The actual reaction is the electrolysis of water.
The reactions are:
2H+(aq) + 2e– → H2(g)
2OH–(aq) → O2(g) + 2H+ + 4e–
Electroplating
Similar to the voltaic cell, metal ions are reduced and deposited on the cathode. To ensure there is no other metal being plated on cathode, the anode electrode is usually inert or made of the same metal as the ions.
Example: Electrolysis of CuSO4 with copper electrodes
Since copper metal is oxidized more easily than hydroxide ions, the anode reaction is the production of copper but not production of oxygen. This replenishes the ions in the electrolyte.
On the cathode, the copper ions are reduced into copper metal, depositing a thin layer of copper on the electrode.
Significance:
If both electrodes are the same metal, impurities in the anode will be eliminated. This is electrorefining.
If the cathode is another type of metal from the electrolyte, the process produces a thin layer of metal deposit on the cathode. This is electroplating.
Marco’s teaching style is interactive and communication-focused; he focuses on motivating students to be active learners. Sign up a trial lesson with Marco now!
About The Edge
The Edge Learning Center is Hong Kong’s premier Test Preparation, Academic Tutoring, and Admissions Consulting services provider. Founded in 2008, The Edge has helped thousands of students improve their ACT and SAT scores as well as their IB and AP grades. The AC team has just finished off another successful period in which students gained acceptance to schools such as Columbia, Yale, UChicago, and more! Check out our latest Admissions Results!

